Orforglipron and tirzepatide, developed by Eli Lilly, are cutting-edge treatments for type 2 diabetes and obesity. While both aim to improve glycemic control and promote weight loss, they differ in formulation, administration, and clinical outcomes.
Mechanism of Action
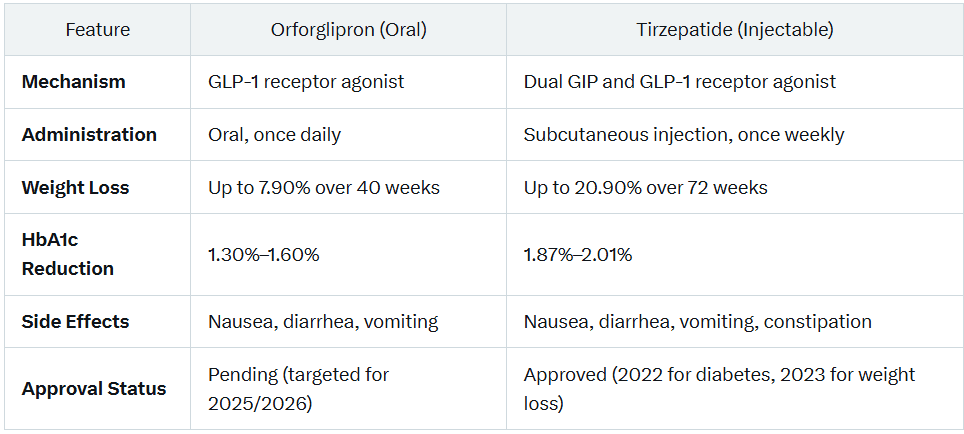
- Orforglipron: A non-peptide, oral glucagon-like peptide-1 receptor agonist (GLP-1 RA). It mimics the GLP-1 hormone to enhance insulin secretion, suppress appetite, and slow gastric emptying (Wharton et al., 2024, Diabetes, Obesity and Metabolism).
- Tirzepatide: A dual agonist targeting glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptors. This dual mechanism improves glycemic control and drives significant weight loss (Jastreboff et al., 2022, The Lancet).
Administration
- Orforglipron: Taken orally once daily, with no food or water restrictions, offering a convenient alternative to injectables (Reuters, 2024).
- Tirzepatide: Administered via subcutaneous injection once weekly, marketed as Mounjaro for type 2 diabetes and Zepbound for weight loss (FDA, 2023).
Efficacy
Note: Trial durations differ (40 weeks for orforglipron vs. 72 weeks for tirzepatide’s weight loss data), limiting direct comparisons.
- Orforglipron:
- Weight Loss: In a 40-week Phase 3 trial, patients with type 2 diabetes achieved up to 7.90% body weight reduction (Eli Lilly, 2024, Phase 3 Trial Data).
- HbA1c Reduction: Reduced HbA1c by 1.30%–1.60% over 40 weeks (Eli Lilly, 2024).
- Tirzepatide:
- Weight Loss: In a 72-week trial, non-diabetic adults lost up to 20.90% of body weight at the highest dose (Jastreboff et al., 2022).
- HbA1c Reduction: Achieved HbA1c reductions of 1.87%–2.01% over 40 weeks (Frias et al., 2021, The Lancet).
Side Effects
- Orforglipron: Common side effects include nausea (13–18%), diarrhea (19–26%), and vomiting (5–14%). No significant liver safety concerns reported (Reuters, 2024).
- Tirzepatide: Common side effects include nausea (15–25%), diarrhea (12–20%), vomiting (8–15%), decreased appetite, constipation, and abdominal discomfort (Frias et al., 2021).
Regulatory Status
- Orforglipron: In Phase 3 trials, with Eli Lilly targeting FDA approval for weight management by late 2025 and type 2 diabetes by 2026. These timelines are tentative and subject to change (Business Insider, 2024).
- Tirzepatide: Approved in the U.S. for type 2 diabetes in May 2022 (Mounjaro) and weight loss in November 2023 (Zepbound). Also approved for obstructive sleep apnea in December 2024 (FDA, 2024).
Summary
Tirzepatide currently outperforms orforglipron in glycemic control and weight reduction, likely due to its dual mechanism and longer trial data. However, orforglipron’s oral administration may improve patient adherence, particularly for those averse to injections. Treatment choice should weigh patient preferences, tolerability, and accessibility. Notably, tirzepatide’s cost varies by region and insurance (approximately $1,000–$1,500/month without coverage), while orforglipron’s pricing remains undisclosed pending approval (Investopedia, 2024).